Water
It is one of the most important compounds which is required for the survival of living organisms on Earth. Water can be found in many water bodies like rivers, oceans ponds, glaciers, lakes, and many others. The main source of water is rainfall and is believed to be the purest form of water due to the fact that there is no salt dissolved in it. However, dissolved gases can be present.
Water can be of two types. It is classified as:
- Soft water: This type of water lathers with soap and it can be obtained from rainfall etc. This type of water can be used for household purposes like cleaning, laundry, and so on.
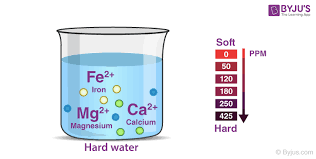
- Hard water: Hard water on the other hand does not lather with soap but rather leads to the formation of a precipitate and this hardness is due to the high content of minerals present in it or other words presence of salts especially magnesium and calcium. These include soluble Chlorides, bicarbonates, and sulfates of magnesium and calcium. This hardness can be determined by the multivalent cations’ concentration present in water. These are metal complexes that are positively charged with a charge of more than 1+. However, most of the cations have a charge of 2+. The widely found cations in hard water are Mg2+ and Ca2+. They enter the water bodies through the leaching process. In contrast to this soft water contains very few ions. The reaction below shows the formation of calcium carbonate:
CaCO3 (s) + CO2 (aq) + H2O (l) ⇋ Ca2+ (aq) + 2HCO3− (aq)
Types of Hardness
There are two types of hardness in water:
- Temporary Hardnes
This type of hardness is mainly due to the presence of calcium and magnesium carbonates in water making it a temporary hardness that can be removed by boiling the water. The hard water can be made soft in the case of soluble salts. For example soluble salt of Mg(HCO3)2 can be converted to Mg(OH)2 that is not soluble forming precipitates that can be removed by filtration, thereby leaving the water as soft water.
- Permanent Hardness
The permanent hardness of water cannot be removed by boiling as the salts present do not precipitate out with an increase in temperature. Therefore hard water is very harmful and not recommended for its use in boilers as they might be deposition of salts that can affect the effectiveness and efficiency of the boiler. Hard water can be used for drinking however, excessive consumption of it can lead to a lot of health problems. When it comes to laundry, spots can appear on clothes. Higher will be the bills as extra work is required for water appliances. It can also cause strains in the skin. The hardness can be removed only when the water is treated with washing soda. The insoluble carbonates are formed after the reaction of washing soda with the sulfide and chloride salts belonging to calcium and magnesium. The insoluble carbonates can be removed making the hard water soft water.
Learn: What causes the temporary and permanent hardness of water?
Disadvantages of Hardness of water
- Due to the hardness of water, there will be a wastage of soap when it comes to laundry, cleaning purposes, etc.
- There will also be wastage of fuel when boiling such type of water.
- Using hard water in metallic boilers can lead to the formation of scales on them.
How can the Hardness of Water be removed?
For Temporary hardness of water:
-
By Boiling:
The bicarbonates that are soluble in water are first converted into insoluble carbonates by boiling which can then be removed by filtration.
Reactions involved is given as under:
Ca(HCO3)2 → ΔCalo3↓ + H2O + CO2
⇒ Mg(HCO3)2 → ΔMgCO3↓ + H2O + CO2
-
By Clarks Method:
A Clark’s reagent is used in this reaction which is Calcium hydroxide. This helps in the removal of the hardness of water which involves the conversion of bicarbonates into carbonate.
Reaction involved:
Ca(OH)2 + Ca(HCO3)2 → 2CaCO3↓ + 2H2O
For Permanent Hardness of Water
-
Gan’s Permutit Method:
Here, sodium aluminium ortho silicate also well-known as zeolite or permutit can be used for the removal of the water’s permanent hardness.
The reaction involved in this reaction:
Na2Al2Si2 O8.KH2O + Ca++→ 2Na+ + Ca Al2 Si2 O8.xH2O
-
Calgon’s Process:
Another method is Calgon’s method, in which the (NaPO3)6, i.e., sodium-hexa-meta-phosphate is used. This is commonly known as Calgon and is used for the removal of the hardness of water by the process of adsorption of Ca++ and Mg++ ions.
Learn: What is Calgon?
-
Ion Exchange Resin Method:
This method is used for the removal of the permanent hardness of water with the help of resins. The Ca++ or the Mg++ ions take part in an exchange reaction where they are first exchanged with Cl–, SO4-2 ions followed by the exchange with RNH2OH ( an anion exchange resin).
⇒ 2RCOOH + Ca++ → (RCOO)2Ca + 2H+
⇒ RNH2OH + Cl– → RNH2Cl + OH–
⇒ H+ + OH– → H2O